Leading Change in Cancer Clinical Research, Because Our Patients Can’t Wait
Updated: 2024-05-31 00:00:00
 Reshaping the cancer clinical trials infrastructure to overcome key bottlenecks will involve embracing technology and collaboration, and inviting innovation, explain NCI Director Dr. W. Kimryn Rathmell and NCI Special Advisor Dr. Shaalan Beg.
Reshaping the cancer clinical trials infrastructure to overcome key bottlenecks will involve embracing technology and collaboration, and inviting innovation, explain NCI Director Dr. W. Kimryn Rathmell and NCI Special Advisor Dr. Shaalan Beg.
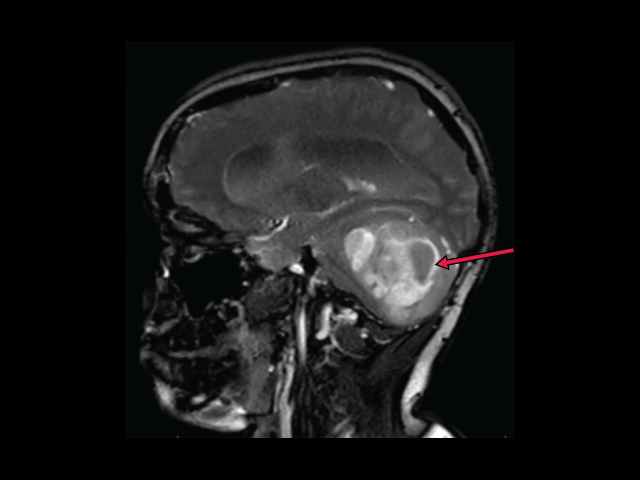 FDA has granted an accelerated approval to tovorafenib (Ojemda) for kids and teens who have low-grade glioma with changes in the BRAF gene. In a small clinical trial, the drug shrank or completely eliminated tumors in nearly 70% of patients.
FDA has granted an accelerated approval to tovorafenib (Ojemda) for kids and teens who have low-grade glioma with changes in the BRAF gene. In a small clinical trial, the drug shrank or completely eliminated tumors in nearly 70% of patients. Assessing and offering people with cancer stepped collaborative care may help better manage symptoms of depression, pain, and fatigue than the standard referral to providers for treatment, according to a recent study.
Assessing and offering people with cancer stepped collaborative care may help better manage symptoms of depression, pain, and fatigue than the standard referral to providers for treatment, according to a recent study.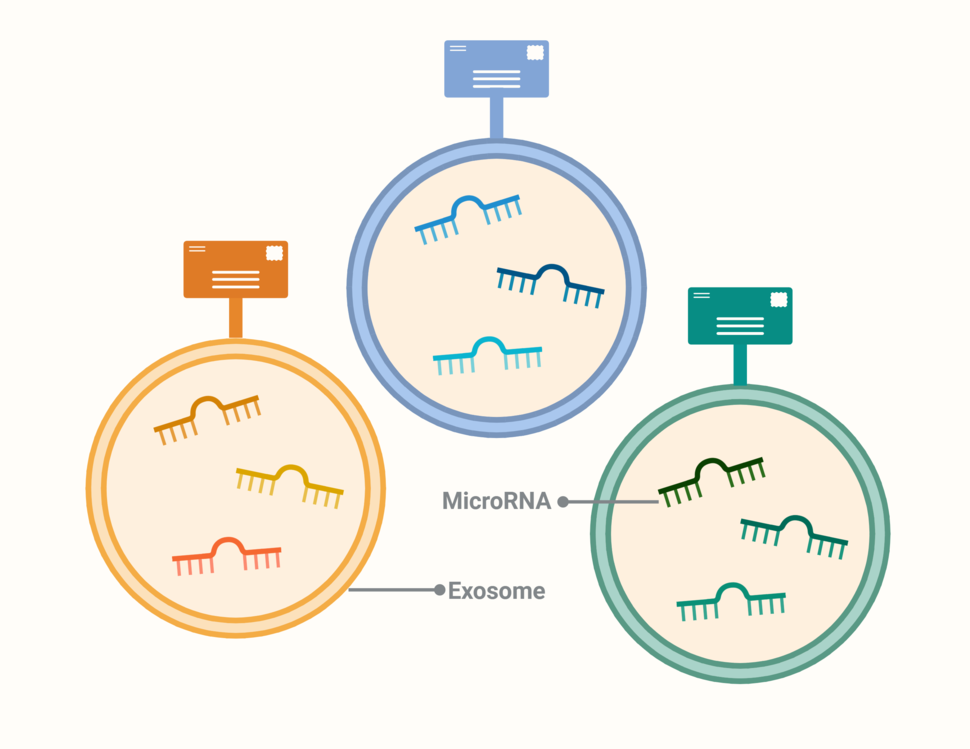 In a new study involving nearly 1,000 people, a liquid biopsy accurately detected early- and late-stage pancreatic cancer. When paired with a test for the protein CA19-9, the combination accurately identified 97% of people with early-stage disease.
In a new study involving nearly 1,000 people, a liquid biopsy accurately detected early- and late-stage pancreatic cancer. When paired with a test for the protein CA19-9, the combination accurately identified 97% of people with early-stage disease.